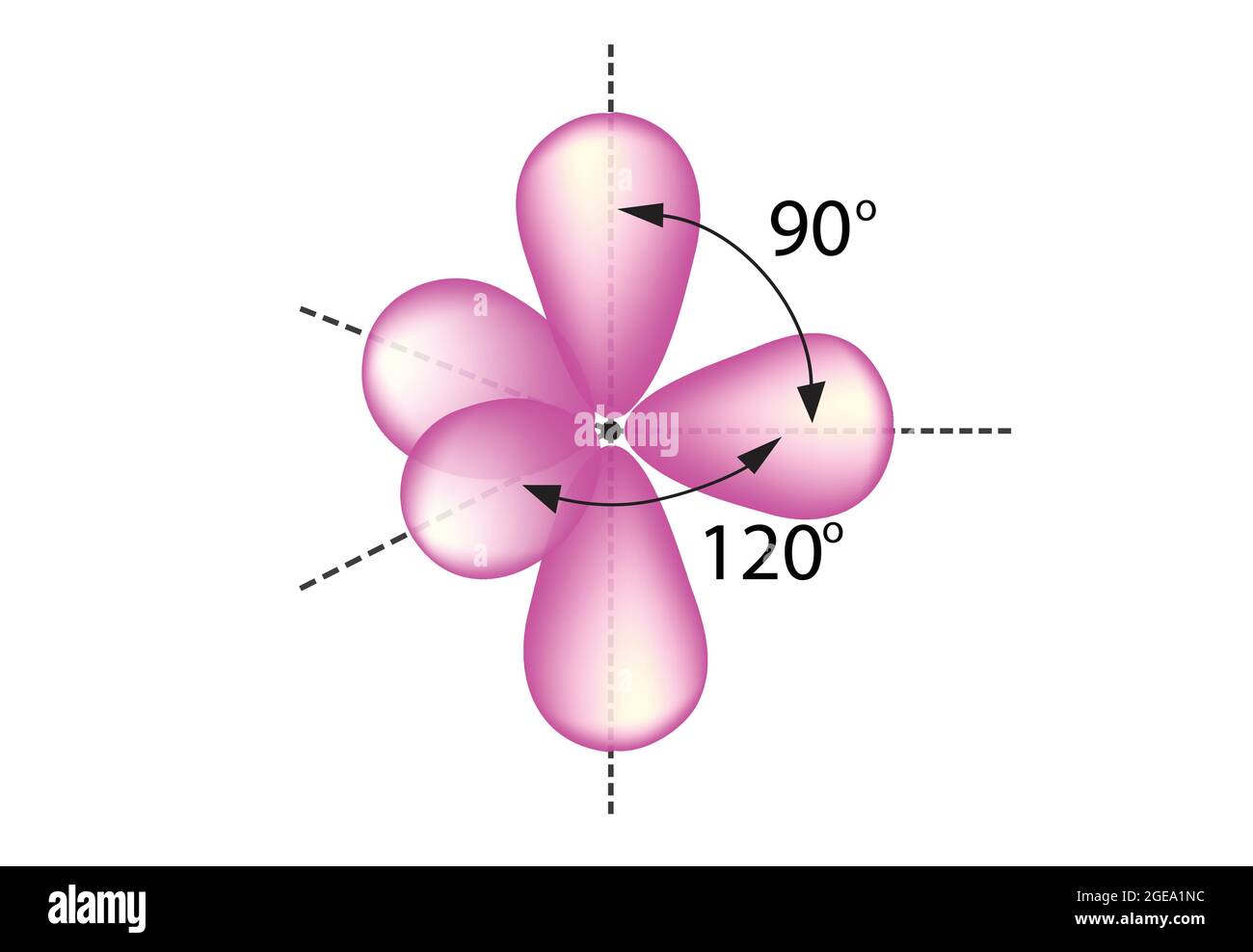
Trigonal bipyramidal arrangement of hybridization, hybrid orbitals, 5 sp3d hybrid orbitals, sp3d Hybridization, angles of 120 and 90 Stock Vector Image & Art - Alamy

Hybridization || sp3d || sp3d2 || sp3d3 || Formation of PF5, SF6 and IF7 || Chemical Bonding 11th - YouTube

Specify the hybridization of the atoms labeled a-d in the following structure and provide your reasoning for each choice. Your options are sp3, dsp2, sp2, sp3d, or sp. Additionally, provide a brief
What are the shapes and bond angles of sp, sp2, sp3, sp3d, sp3d2 hybridised orbitals respectively? - Quora

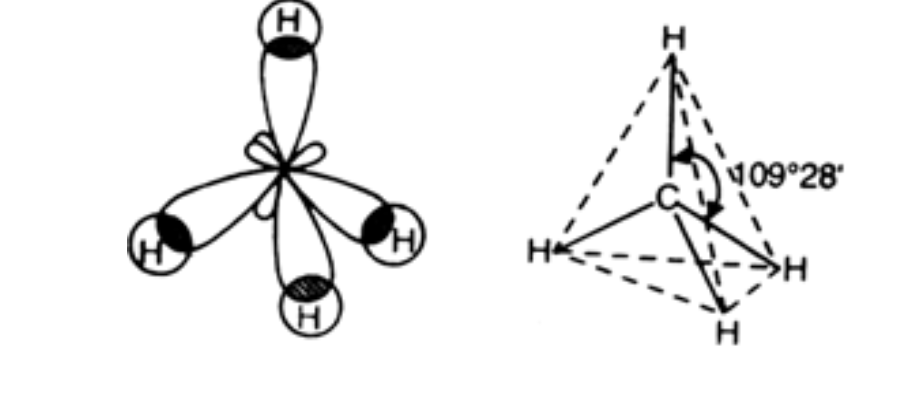
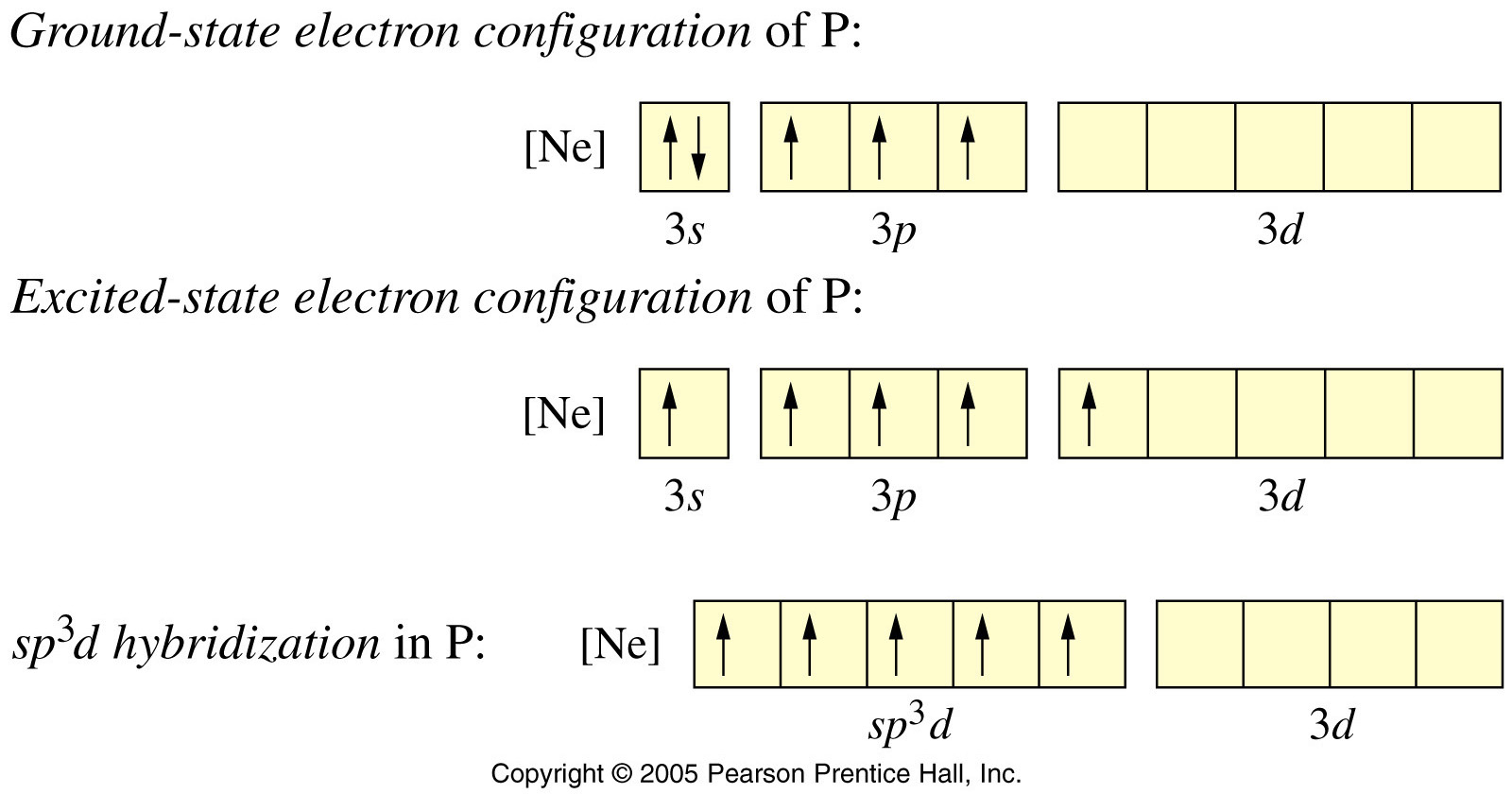




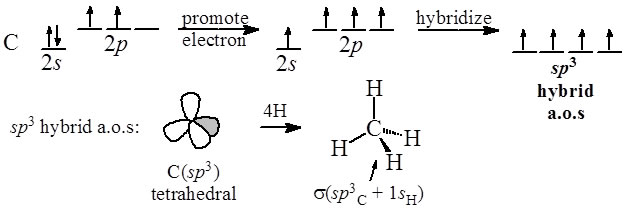
![Sp3d hybridization 3d model - Download Free 3D model by AK Chemistry (@AKchemistry) [2e70339] Sp3d hybridization 3d model - Download Free 3D model by AK Chemistry (@AKchemistry) [2e70339]](https://media.sketchfab.com/models/2e70339904ab4a749cd354848a2899ea/thumbnails/05fa22e5565e4c278fa48b79987f7ea0/91522085ccc34923a3f5573359e3b951.jpeg)
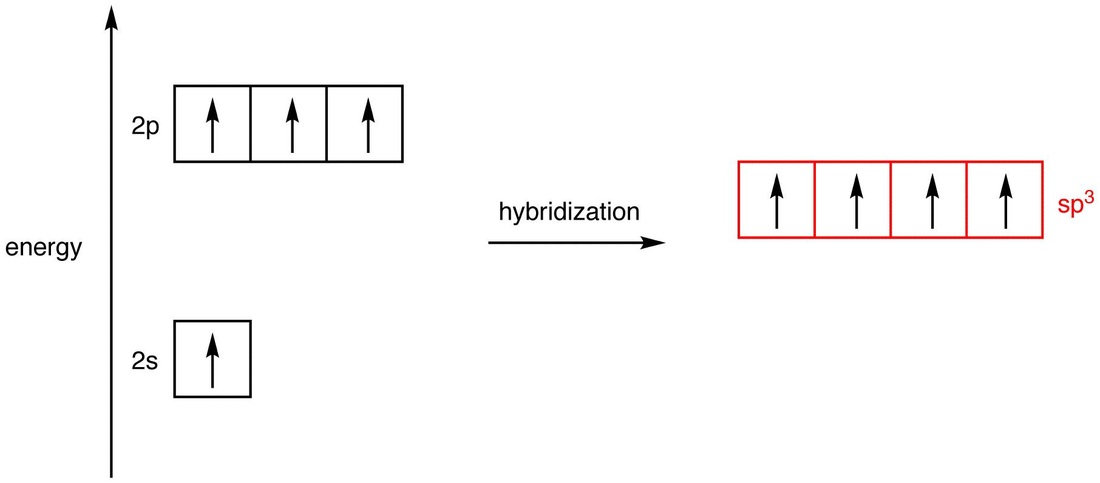







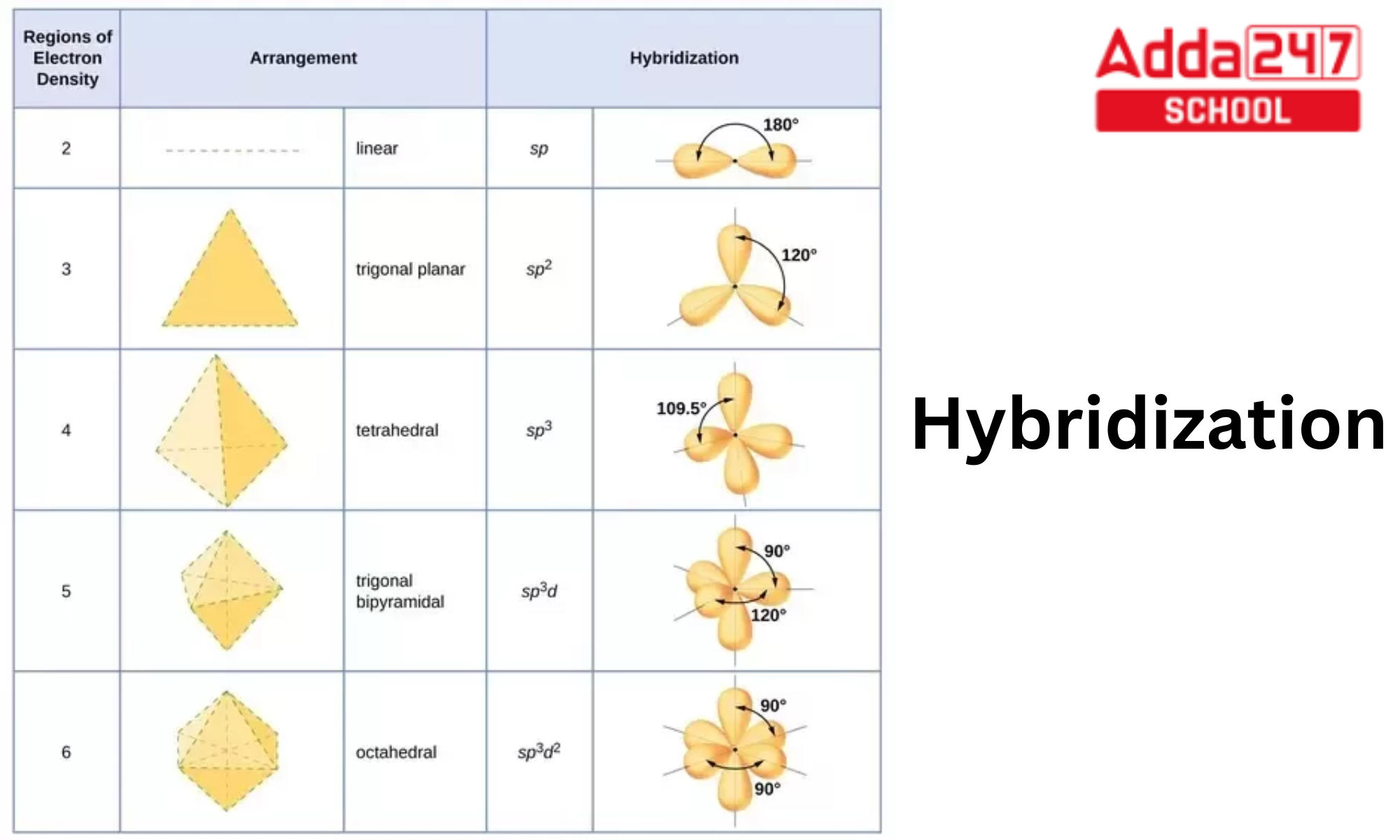

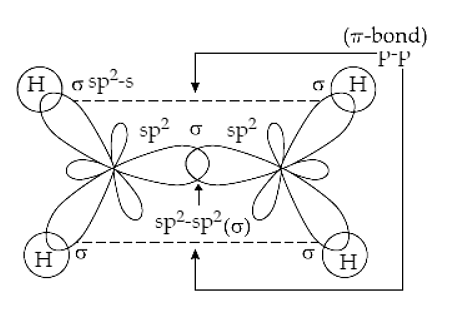
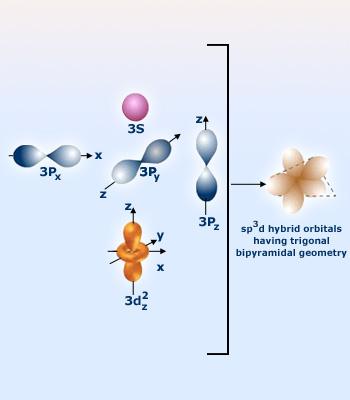
![Explain \\[s{p^3}d\\] hybridization by taking a suitable example. Explain \\[s{p^3}d\\] hybridization by taking a suitable example.](https://www.vedantu.com/question-sets/0801a856-ce79-45dd-96b3-f5dcbf552a724106220300996909947.png)